Motonori HOSHI, D.Sc.
The Open University of Japan (The University of The Air) |
|
Motonori Hoshi is currently a Professor of the Open University of Japan (The University of The Air). Dr. Hoshi received his doctorate in Science in 1970 at the University of Tokyo. While serving as an Instructor at the Department of Biology, University of Tokyo, he also spent his postdoctoral fellowship at the Department of Neurology, Massachusetts General Hospital/Eunice Kennedy Shriver Center for Mental Retardation (1970-1972). He was assigned Associate Professor at the Institute of Low Temperature Sciences, Hokkaido University (1973-1981) and then Department of Biology, Nagoya University (1981-1984). He was then appointed Professor for the Biological Laboratory/Department of Life Science, Tokyo Institute of Technology in (1985-2000), and later he became Professor of Department of Chemistry/Department of Biosciences and Informatics, Keio University (2000-2006).
Dr. Hoshi has served on numerous scientific, advisory and editorial boards and is currently a Senior Academic Advisor of the Japan Society for the Promotion of Science.
Additionally, he served as President of the International Society of Invertebrate Reproduction (1990-1992), Zoological Society of Japan (1999-2002), and International Union of Biological Sciences (2004-2007).
He won Mizutani research grant in 1998.
|
|
|
|

his is a much biased view of glycoscience and glyco-world by a biologist who has been studying animal development and reproduction focusing on the role of glycoconjugates.
 Life is equally expressed in unity and diversity. Indeed, Edward O. Wilson (1) wrote, "The most wonderful mystery of life may well be the means by which it created so much diversity from so little physical matter". Similarly, Edwin Chargaff (2) warned us as follows: "The edifice of the animated world rests, one could say, on two pillars: one is the unity of nature, the other is diversity. To pay attention only to the unity, as is usually done, completely distorts our vision and condemns us to the kind of analogy research that fills our journals". The central dogma expresses the first approximation of life in which the players are only semantides (semantophoretic molecules) (3), namely nucleic acids and proteins. Although the backbone of nucleic acids is constructed of deoxyribose phosphate or ribose phosphate, these sugars do not directly bear genetic information. However, if you look at life more closely, you will find that important biological information is carried also by episemantides (episemantic molecules) such as sugars and lipids. Sugars give cells biological information and finely tuned environments besides the energy and structural rigidity. The semantides are linear polymers and their basic structures are rather simple. They are synthesized as complementary copies by using parent molecules as a mold, and a single mechanism can make many different molecules if different molds are used. This feature of semantides constitutes the basis for gene technologies. Sugar chains are not necessarily linear and often very complicated, and they are not synthesized as copies. Instrumental analysis and synthesis of sugar chains have been much improved, yet it is not an easy task to determine the structure of sugar chains and synthesize them, compared to the sequencing and synthesizing of semantides. This is a serious problem that needs to be overcome in glycoscience. Life is equally expressed in unity and diversity. Indeed, Edward O. Wilson (1) wrote, "The most wonderful mystery of life may well be the means by which it created so much diversity from so little physical matter". Similarly, Edwin Chargaff (2) warned us as follows: "The edifice of the animated world rests, one could say, on two pillars: one is the unity of nature, the other is diversity. To pay attention only to the unity, as is usually done, completely distorts our vision and condemns us to the kind of analogy research that fills our journals". The central dogma expresses the first approximation of life in which the players are only semantides (semantophoretic molecules) (3), namely nucleic acids and proteins. Although the backbone of nucleic acids is constructed of deoxyribose phosphate or ribose phosphate, these sugars do not directly bear genetic information. However, if you look at life more closely, you will find that important biological information is carried also by episemantides (episemantic molecules) such as sugars and lipids. Sugars give cells biological information and finely tuned environments besides the energy and structural rigidity. The semantides are linear polymers and their basic structures are rather simple. They are synthesized as complementary copies by using parent molecules as a mold, and a single mechanism can make many different molecules if different molds are used. This feature of semantides constitutes the basis for gene technologies. Sugar chains are not necessarily linear and often very complicated, and they are not synthesized as copies. Instrumental analysis and synthesis of sugar chains have been much improved, yet it is not an easy task to determine the structure of sugar chains and synthesize them, compared to the sequencing and synthesizing of semantides. This is a serious problem that needs to be overcome in glycoscience.
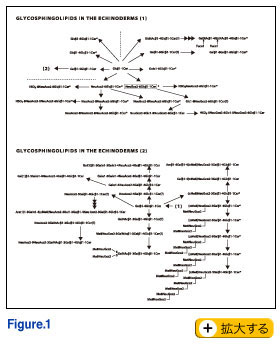
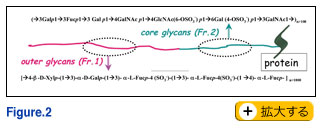 In the field of Developmental Biology, Evo-Devo has recently become oft-spoken catch phrase, and the environment is often ignored even though development is deeply influenced by it. A single genotype can produce many phenotypes, depending on the many contingencies encountered during development (4). This ignoring of the environment may be caused by too much preference given to a small number of "model systems" used as material. Each species plays a species-specific variation of the same music, the music of life. Each variation may clearly express an important theme which might be not obvious in other variations. We must remember that many important phenomena and concepts have been discovered and established by using "strange" organisms. In this context, I am afraid that glycoscience is not being explored broadly enough in various groups of organisms. Even within my limited experience, I realized that, in the echinoderm gametes, most glycolipids, especially gangliosides, have quite unique structures as shown in Fig 1. The quantity of gangliosides in sea urchin eggs is exceptionally high. We also found, in starfish eggs, that a proteoglycan-like molecule which carries important information for sperm has repeating units of pentasaccharide and of heptasaccharide. The biological information is mostly attributable to the sugar chains.
Finally, I would like to point out that microheterogeneity in glycoproteins and proteoglycans may not be a sort of errors in the synthetic process as generally believed, but the cells may make such "errors" on purpose in an effort to fine tune their response to environmental changes. In other words, sugar chains can provide much more subtly different conditions with much less cost than the semantides. In the field of Developmental Biology, Evo-Devo has recently become oft-spoken catch phrase, and the environment is often ignored even though development is deeply influenced by it. A single genotype can produce many phenotypes, depending on the many contingencies encountered during development (4). This ignoring of the environment may be caused by too much preference given to a small number of "model systems" used as material. Each species plays a species-specific variation of the same music, the music of life. Each variation may clearly express an important theme which might be not obvious in other variations. We must remember that many important phenomena and concepts have been discovered and established by using "strange" organisms. In this context, I am afraid that glycoscience is not being explored broadly enough in various groups of organisms. Even within my limited experience, I realized that, in the echinoderm gametes, most glycolipids, especially gangliosides, have quite unique structures as shown in Fig 1. The quantity of gangliosides in sea urchin eggs is exceptionally high. We also found, in starfish eggs, that a proteoglycan-like molecule which carries important information for sperm has repeating units of pentasaccharide and of heptasaccharide. The biological information is mostly attributable to the sugar chains.
Finally, I would like to point out that microheterogeneity in glycoproteins and proteoglycans may not be a sort of errors in the synthetic process as generally believed, but the cells may make such "errors" on purpose in an effort to fine tune their response to environmental changes. In other words, sugar chains can provide much more subtly different conditions with much less cost than the semantides.
I believe that technological innovations in the near future will allow us to recognize the glyco-world as precisely as the cells do. When that happens we will be able to understand the meaning of the "stranges" phenomena often found in glycoscience now.
|
|
 |
|
Reference: |
|
| 1. |
Wilson E. O. The Diversity of Life, Harvard University Press, 1992 |
| 2. |
Chargaff E. HERACLITEAN FIRE Sketches from a Life Before Nature, The Rockefeller University Press, 1978 |
| 3. |
Zuckerkandl E, Pauling L. Molecules as documents of evolutionary history. J. Theor. Biol. (8): 357-366, 1965 |
| 4. |
Nijhout H. F. Polyphenic Development in Insects In polyphenic development, environmental factors alter some aspect of development in an orderly and predictable way. BioScience 49: 181-192, 1999 |
|
|


